With the health and wellbeing of patients at stake, any organization that is certified as a marketing authorization holder for pharmaceuticals must commit to product quality assurance and safety management. In Japan, as in many countries around the globe, management of the quality and safety of pharmaceutical products must be carried out in accordance with the law* and regulations enforced by the Ministry of Health, Labour and Welfare. It is essential for pharmaceutical companies who are marketing authorization holders to have a system in place that ensures total compliance.
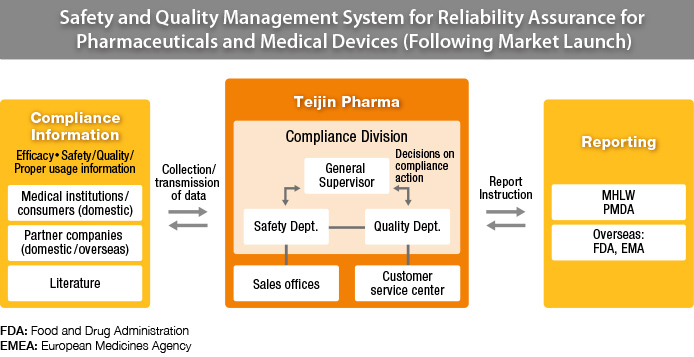
The regulations stipulate the following: Under a general supervisor, the pharmaceutical company must have a quality assurance department and prepare a quality standard code which contains up to date information on each of their products, including drug composition and manufacturing methods. A quality assurance duty procedure document containing details on procedures such as manufacturing control, quality assurance, market release, storage and training must be prepared. Vigilance practices must be in place to continuously manage feedback. A safety control management department is necessary, also under the purview of the general supervisor, where safety control information is gathered from medical institutions and partner companies. All feedback, including things such as adverse reactions or ineffectiveness, must be evaluated.
*The Law on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices
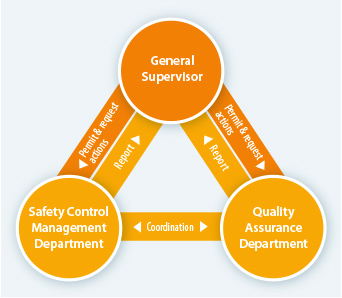
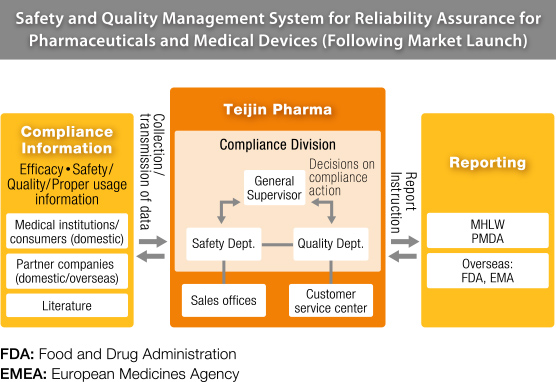
As a marketing authorization holder, Teijin Pharma has a well-established system in place. Our Compliance Division operates independently from other parts of the company, under the direct control of the president. This way, when an issue is reported it can be responded to quickly and neutrally. The division can liaise immediately with the company president on an issue, then go directly to the relevant departments to request any actions needed.
Members of the Compliance Division have ample experience in their fields, including R&D, production and sales (as medical representatives). Members in these roles prioritize communication, maintaining close relationships with all departments inside Teijin Pharma. There are two Quality Assurance departments inside the Compliance Division that deal with the quality assurance of pharmaceuticals and medical devices respectively. These two departments manage and supervise all stages of production, from R&D to post-sale. In addition to these two, the Medical & Pharmaceutical Information Department manages and analyzes all data regarding safety and proper usage of Teijin Pharma products. The Regulatory Affairs Department handles the documentation required to receive approval for commercial release of our products and provide planning for how Teijin Pharma can reliably comply with laws, regulations and other legal standards. Lastly, the Approval Application Maintenance Section ensures that all pharmaceuticals leaving Teijin Pharma have been manufactured in compliance with the law, regulations and our own high standards.
Under the regulations set by the Ministry of Health, Labour and Welfare, it is necessary for anyone working in a post-sale products safety management role to participate in ongoing education and training, as well as self-auditing. At Teijin Pharma, our Compliance Division handles this too. The Medical & Pharmaceutical Information Department provide relevant educational opportunities for all medical representatives (MRs), providing online questionnaires that act as both educational materials and self-auditing tools. Both quality assurance departments, one focused on pharmaceuticals/drugs, the other on medical devices, provide educational lectures on regulatory compliance relevant to their respective fields.
On top of these ongoing educational programs, we also hold annual lectures for all Teijin Pharma members, covering topics that are relevant to all. In 2018, the topic was drug-induced suffering. Introducing historic cases in Japan, such as the mishandling of blood products leading to patients contracting hepatitis C, the lecture highlighted preventable cases of drug mishandling. In the hepatitis C outbreak case, many patients were affected and the resulting lawsuit led to much stricter laws and regulations. Through studying these historic cases, members can better understand the importance of compiling data from around the world to strengthen our safety measures. In 2019, the topic was counterfeit medicinal products, discussing the rising volumes of drugs that are imported to Japan through unregulated channels. Customers are sometimes duped into buying counterfeit products slipped in with officially certified products, underlining the importance of managing raw materials, packaging materials and controlling where our products go.

Any drug or medical device that has been certified for market launch has been through several phases of clinical trials. However once products are made available to patients, unforeseen results or adverse reactions may still appear. Pharmaceuticals must be consistently reassessed for risk both before and after general sale to the public. Compiling safety control information is vital to minimize risk to patients. Teijin Pharma proactively gathers information from medical professionals, academic research and partner companies both in Japan and around the world. The data collected is compiled into a database that is shared with our main partners globally. When a drug is first released, the incoming data might reach thousands of reports in a single month. Our Compliance Division analyses each of these reports for their severity and cause and, when necessary, distributes the information to medical professionals through medical representatives (MRs) or revises package inserts. We also share information with medical professions online via the Pharmaceutical and Medical Devices Agency in the form of risk management plans outlining safety specifications and risk minimization for each product.
Complying with the law is necessary, but so is providing medical products that give patients the treatments that they need while maintaining their quality of life. To provide this support, we must have confidence in our ability to effectively manage risk for patients.
Related Links
- Project Stories Vol. 11 Innovative Rehabilitation Devices for Stroke Patients
- Project Stories Vol. 10 Enabling First Class Care at Home
- Project Stories Vol. 9 Celebrating Teijin Pharma’s History as a Core Part of Teijin
- Project Stories Vol. 8 Expanding the Range of Treatments Available to Patients of Acromegaly
- Project Stories Vol. 7 Enriching the Lives of Patients through Our Community
- Project Stories Vol. 6 Building a new culture of healthcare through innovation
- Project Stories Vol. 5 Creating Products to Match Medical Needs
- Project Stories Vol. 4 Improving Quality of Life for SAS Patients
- Project Stories Vol. 3 Building a Strong Platform in the Field of Bone and Joint Diseases
- Project Stories Vol. 2 Providing Home Healthcare Support in a Disaster
- Project Stories Vol. 1 A New Drug Born from R&D