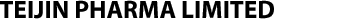Jun. 05, 2019
Teijin Pharma Starts to Exclusive Sales of Transcranial Magnetic Stimulation Device in Japan
Teijin Pharma Limited
Teijin Pharma Limited, the core company of the Teijin Group’s healthcare business, announced today that it starts exclusive sales of NeuroStar® Transcranial Magnetic Stimulation (TMS) in 5 June, which was first listed under coverage of Japanese national health insurance as the TMS device for depression on 1 June. Teijin Pharma signed an exclusive sales agreement in Japan with Neuronetics, Inc.,the U.S. medical device maker in 2017.
According to the patient survey of Ministry of Health, Labor and Welfare, Japan has some 730,000 patients receiving treatment for depression and as many as 2.5 million including up to potential depression patients, of which approximately 30% are said to have treatment-resistant depression. To better address the situation, there is a growing need for safe new therapies since current augmentation drug therapies that combine agents with different mechanisms of action, and electroconvulsive therapies that apply electrical current to the head, carry side-effect risks although they are effective.
The NeuroStar® TMS was cleared by the U.S. Food and Drug Administration as the nation’s first TMS device for the treatment of depression in 2008, with 10 years’ experience for about 60,000 patients. The NeuroStar® is TMS device for depression that does not respond well to normal antidepressant drug-based therapies, only listed under coverage of Japanese national health insurance. The NeuroStar® TMS produces an electrical current in the brain which elicits action potentials and release of neurotransmitters1, and it has been proven effective in reducing or eliminating symptoms of depression by magnetically stimulating the brain’s left dorsolateral prefrontal cortex using it from 20 to 30 times for 5days a week2.The NeuroStar® TMS is used in an advanced therapy, a noninvasive magnetic-stimulus system that provides benefits without the side effects often associated with antidepressant medication. Most common side effect of this device is discomfort at or near the treatment site, and it will be expected to be the new option to the patients.
- 1 Post, A and Keck, ME(2001). J Psychiatr Res
- 2 Kito, S, Fujita,K, and Koga, Y (2008). Neuropsychobiology
An organizer of this device is required to participate in both TMS practical training sessions held by the Japanese Society of Psychiatry and Neurology, and by Teijin pharma.
| Product name | NeuroStar®TMS Therapy for Depression |
|---|---|
| Common name | Transcranial Magnetic Stimulation Device |
| Purpose and effectiveness | Treatment for depression in adults (limited to the patients who does not respond well to normal antidepressant drug-based therapies) |
| Medical device approval number | 22900BZI00029000 |
| Classification | Specially controlled medical device Medical equipment requiring specialist maintenance |
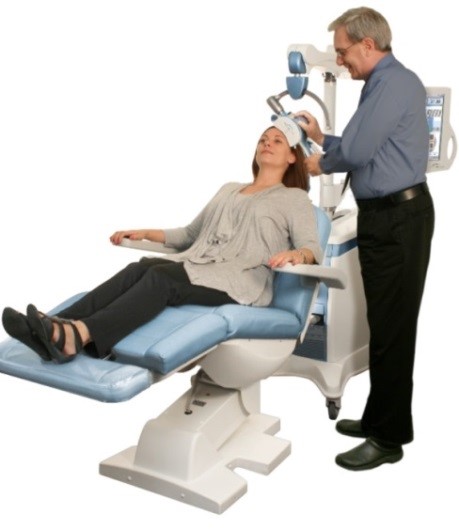
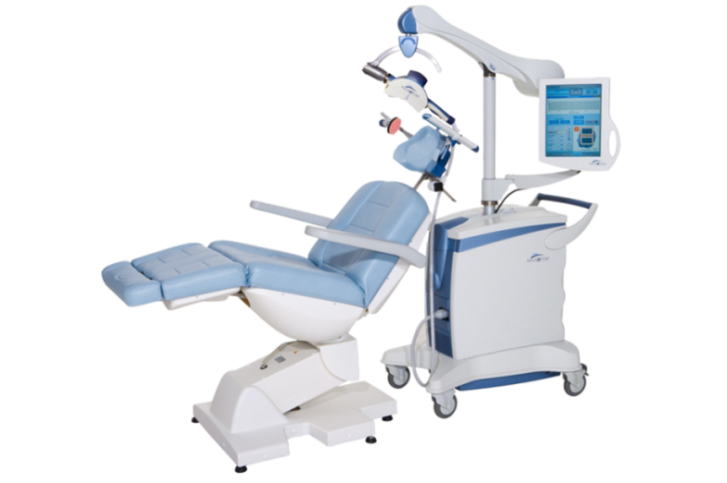
NeuroStar TMS Therapy for Depression
Teijin Pharma promotes appropriate usage of NeuroStar® TMS with related conferences and medical specialist, contributing alleviation of symptoms and improvement of quality of life for depression patient. Teijin, as one of its key growth strategies, is diversifying its healthcare product and service lineups, and is developing innovative medical devices focusing on cranial nerve diseases and rehabilitation fields.
About the Teijin Group
Teijin (TSE: 3401) is a technology-driven global group offering advanced solutions in the areas of environmental value; safety, security and disaster mitigation; and demographic change and increased health consciousness. Its main fields of operation are high-performance fibers such as aramid, carbon fibers & composites, healthcare, films, resin & plastic processing, polyester fibers, products converting and IT. The group has over 170 companies and around 20,000 employees spread out over 20 countries worldwide. It posted consolidated sales of JPY888.6 billion (USD 8.1 billion) and total assets of JPY 1020.7 billion (USD 9.3 billion) in the fiscal year ending March 31, 2019. Please visit www.teijin.com
Press Contact
Corporate Communications
Teijin Limited
+81 3 3506 4055
pr@teijin.co.jp