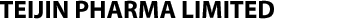Research & Development 
Research & Development 
Teijin Tokyo Research Center - A place where diverse knowledge comes together -
Teijin Pharma's research laboratory is located at the Teijin Tokyo Research Center in Hino City, in the western part of Tokyo. Here, we conduct research activities aimed at creating new healthcare solutions, based on the drug discovery and medical technology research we have cultivated thus far.

In April 2024, Axcelead Tokyo West Partners, Inc. (ATWP), a joint venture between Teijin Ltd. and Axcelead, Inc., was established, taking over Teijin Pharma‘s wet research functions*1 and beginning its business as a PRO (Partnering Research Organization). Teijin Pharma will maintain its dry research functions*2 and continue to pursue drug discovery research in collaboration with ATWP.
*2 Creation and promotion of new research programs, utilization of computer science including AI
The same premises also house the R&D departments of regenerative medicine, implantable medical devices, and the functional foods business in the Teijin Group. The sharing of information and knowledge between organizations, which occurs both formally and informally, is fostering an environment in which experts from various fields can inspire each other.
Our R&D Structure utilizing the Drug Discovery Ecosystem
Teijin Pharma will contribute to improving the Quality of Life of patients in Japan and overseas by utilizing the "Drug Discovery Ecosystem," in which we collaborate with various R&D partners, not just those within ATWP and the Teijin Group.
Through cooperation with domestic and international drug discovery CROs (Contract Research Organizations), academia, startups, and large and medium-sized pharmaceutical companies, we aim to improve the efficiency and speed of drug discovery research and development and bring innovative drugs to the market as soon as possible.
Teijin Pharma is open to joint research and development, even from the early discovery research stages. We recognize that the greatest innovations that meet the needs of patients are not reached alone but through effective partnering. Our strategy is to seek partners that allow us to combine our innovative science, technologies, and infrastructures with their research, development, manufacturing, and commercial capabilities, in order to provide breakthrough solutions rapidly and efficiently. We believe we can create new value by maximizing our mutual strengths through collaboration or alliance.
Licensing/Alliance in Pharmaceutical Research and Development
Teijin Pharma has a history of successful long-term relationships around the world with strategic partners, ranging from domestic academic researchers to start-up ventures and global companies.
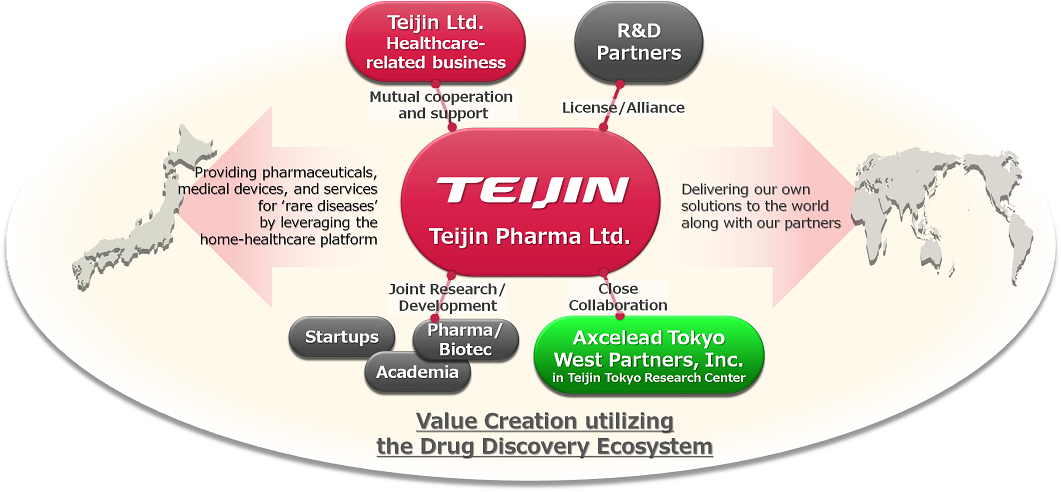
In order to deliver the solutions created through our in-house research to patients around the world, we have actively out-licensed our products to overseas companies. Febuxostat, a gout and hyperuricemia drug created by our laboratory, has become a global product that has been out-licensed to overseas companies and has been marketed in 78 countries, including the United States and European countries. We also focus on out-licensing at the discovery research stage, and have closed multiple deals with large and medium-sized overseas companies.
For more information, please see the news releases for out-licensing of Alzheimer’s disease, kidney disease, narcolepsy and rheumatoid arthritis candidates.
Teijin Pharma is also actively promoting joint research with research institutes and companies both in Japan and overseas to obtain new drug discovery technologies and to accelerate research and development.
Introducing Overseas Innovation to the Japanese Market
We are continually in-licensing products and pipeline items from overseas pharmaceutical companies for the Japanese market. In recent years Teijin Pharma has been chosen by licensors as a development and sales partner for rare and intractable disease programs in the Japanese market. Through steady development in compliance with Japan's regulatory environment, Teijin Pharma is delivering new medicines created by overseas partners to Japanese patients. We also accurately identify unmet medical needs in Japan and promote development for expanding indications, leading to maximization of the product value.
Teijin Pharma continues to seek out partnership opportunities to provide excellent overseas healthcare solutions to Japanese patients suffering from rare and intractable diseases.
Providing Safe and Secure Medical Care at Home - Development of home medical equipment and services –
Teijin Pharma aims to provide safe and secure medical care at home, like the care it offers in a hospital, leveraging the business foundations it has built up through its pharmaceutical and home healthcare businesses.
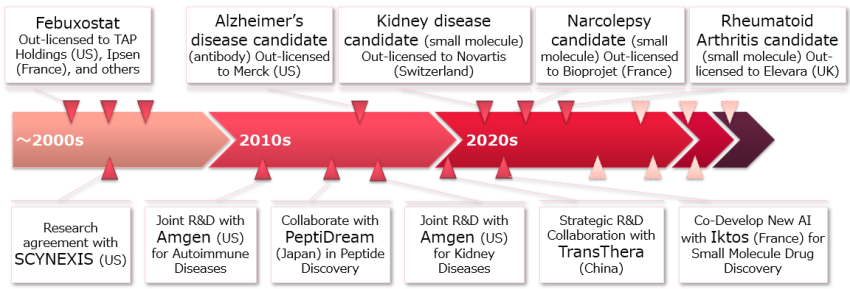
The oxygen concentrators used in home oxygen therapy (HOT), one of Teijin Pharma's core businesses, originate from basic research in Teijin's materials business, and were developed and commercialized in the pharmaceutical business. Even after commercialization, we have developed energy-saving, highly reliable process control technologies, low-noise, low-vibration design technologies, and usability design technologies, and have incorporated these technologies into our device development to enable our customers to use advanced medical equipment comfortably and safely at home.
The research laboratory also strives to create novel healthcare solutions by leveraging our expertise in medical technology and engineering. Beyond developing equipment, we aim to provide compassionate medical care that is responsive to the challenges and suffering experienced by the patient. This includes developing and implementing systems to monitor the status of medical care provided at home and offering services to respond to emergency situations.
Teijin Pharma is constantly searching for advanced medical equipment from overseas and introducing home medical equipment in Japan, where there are high unmet needs. Drawing on the knowledge and expertise we have cultivated as a leading provider of home healthcare, we have successfully adapted commercialized products from overseas to suit the Japanese market, and are committed to continuing this effort.
Consideration of laboratory animal welfare in research and development
In the process of developing pharmaceuticals that contribute to enhancing the quality of life, research procedures using laboratory animals are essential to confirm the safety and efficacy of the pharmaceuticals under development. When conducting such procedures, we apply the three Rs (Replacement, Reduction, and Refinement), the internationally accepted approach to the use of laboratory animals.
Specifically, we have established institutional regulations regarding animal research procedures in compliance with the national regulations on animal welfare enforced by Implementing Agencies under the jurisdiction of the Ministry of Health, Labour and Welfare (MHLW); the Act on Welfare and Management of Animals, the Fundamental Guidelines for Proper Conduct of Animal Experiments, etc., and other relevant laws and guidelines. We also offer continual education opportunities to personnel to ensure the institutional regulations. We have organized the Institutional Animal Care and Use Committee (IACUC) for the purpose of reviewing and approving the design of animal research procedures conducted at our institution. The IACUC members must maintain the confidentiality of animal research procedures and ensure information security. In addition, the IACUC regularly conducts facility and laboratory inspection, and reviews animal care and use to ensure that animal research procedures are always conducted appropriately.
By our efforts described above, we have been accredited by the Animal Testing Facility Certification Center of the Japan Pharmaceutical Information Center for meeting the guidelines of the MHLW.
Production that ensures high quality and stable supply: Industrial Technology